Photoelectrochemical water splitting and CO2 conversion based on 3C-SiC materials; Ga2O3 solar-blind Photodetectors and Transistors.
- Photoelectrochemical Water Splitting and CO2 conversion
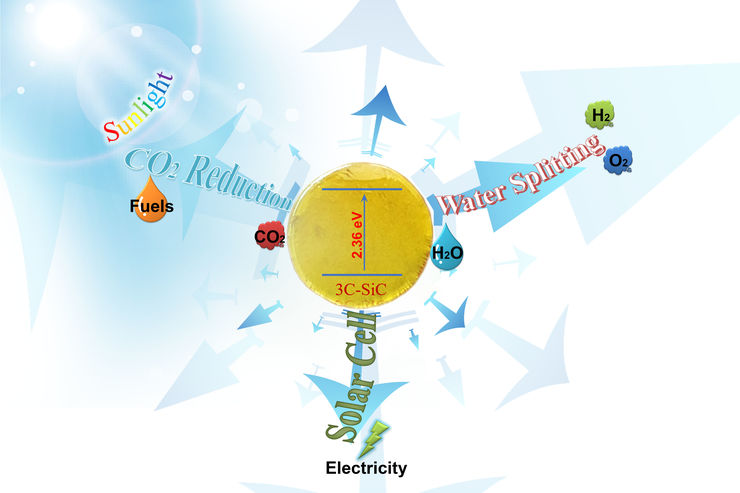
Photoelectrochemical (PEC) water-splitting offers a promising method to convert the intermittent solar energy into renewable and storable chemical energy. We focus on the development of cubic silicon carbide (3C-SiC) semiconductor as the photoelectrode for the solar-to-fuel conversion. 3C-SiC is a promising semiconductor for PEC water splitting due to its relatively small bandgap of 2.36 eV, which is close to the hypothetical ideal bandgap of 2.03 eV for the theoretical maximum of the solar water splitting efficiency. Most importantly, the conduction and valence band positions of 3C-SiC ideally straddle the water redox potentials.We also aim to tailor the properties of 3C-SiC by combination with other catalytic materials for improving the PEC conversion efficiency.
- Ga2O3 Solar-blind Photodetectors and transistors
Ultraviolet (UV) photodetectors, in particular solar-blind photodetectors, have attract much attention because of its wide civil and military applications such as in non-line-of-sight optical communication, fire detection, chemical/biological analysis, and UV astronomy, etc. Gallium oxide, as a wide bandgap semiconductor material, has recently attracted much research interest because of its excellent physical properties such as the wide bandgap of ~4.9 eV and the extremely large breakdown electric field of 8 MV/cm. These promising material properties enable a wide device application prospect in transistor, solar-blind photodetector. Monoclinic phase gallium oxide (β-Ga2O3) is the most chemically and thermally stable material at room temperature among its five existing crystalline structures (α, β, γ, δ, and ε). Recently, it has been demonstrated that high quality 2 inch of gallium oxide single crystal can be grown. And it triggers a hot research field of solar-blind photodetectors and transistors based on gallium oxide.

