The prion diseases Creutzfeldt-Jakob’s disease and Bovine Spongiform Encephalopathy (Mad cow disease) are often associated with amyloid deposition. The term Prion is an abbreviation for PRotein Infectious ONly. Prions, in the classic sense, are protein infectious agents composed of the prion protein PrP. However, the debate is ongoing regarding prion properties also exhibited by amyloid assemblies comprised of other proteins.
The prion protein particle was the first example of an infectious agent that lacks nucleic acid, as opposed to conventional infectious agents such as parasites, bacteria or viruses. Prions cause a variety of diseases in humans and animals including bovine spongiform encephalopathy (BSE) or mad cow disease, Creutzfeldt-Jakob disease (in humans), Chronic wasting disease (in deer and elk) and scrapie (in sheep). Collectively prion diseases are in medical terms named transmissible spongiform encephalopathies or TSE´s due to the transmissibility of the diseases and the pathological feature of microvacuole formation in affected areas of the brain.
A dramatic feature of the transmissible spongiform encephalopathies, TSEs, is the rapid and efficient neuronal degeneration process. Misfolding of the prion protein (PrP) is a crucial step in these diseases. Here, the PrP misfolding process entails the conversion of a helical protein to a largely insoluble β–sheet rich state.
But, how can a misfolding process be infectious? The novel and controversial concept of the prion hypothesis is the propagation of protein conformations through structure based templated folding presented by Stanley Prusiner and is replicated in vitro (see Figure below).
PrP - The full-length soluble domain of mature human PrP protein is composed of 209 amino acids (res. 23-231) and is, in the natively folded PrPC form, folded into two domains. The N-terminal unstructured domain comprising the initial 90 residues has very high flexibility and affinity for Cu2+. The C-terminal globular domain folds into four helices and two short β–strands. The structure of the disease causing and infectious misfolded PrPSC particle is not known in detail due to the insolubility of the protein. PrPSC adopts a stable oligomeric structure with a large amount of β–sheet structure. Prion strains appear to be encoded in the PrPSC structure.
 The prion protein can convert into its disease causing amyloid conformation both through a sporadic route and by templated seeding by adding a small fraction already formed amyloids. The spontaneous, unseeded reaction is significantly slower than the seeded reaction. Image adapted from Nyström et al, Journal of Biological Chemistry 2012 http://www.jbc.org/content/287/31/25975.long
The prion protein can convert into its disease causing amyloid conformation both through a sporadic route and by templated seeding by adding a small fraction already formed amyloids. The spontaneous, unseeded reaction is significantly slower than the seeded reaction. Image adapted from Nyström et al, Journal of Biological Chemistry 2012 http://www.jbc.org/content/287/31/25975.long
Prion disease in mammals
Prion diseases, also termed TSEs (transmissible spongiform encephalopathy) are known to afflict a large number of mammals. The disease is mediated either by sporadic events of misfolding of the endogenously expressed PrP, or by transmission of preformed, misfolded prion particles from a source outside of the host.
BSE in cattle, Scrapie in sheep, MSE in mink and Chronic wasting disease in wild cervids has been known for several decades. More recently, prion disease has been discovered in dromedary camels in North Africa, demonstrating again the wide spread of prion disease among mammalian species.
The three-dimensional fold of native PrP from a wide range of mammals is well conserved. However, the amino acid sequence can vary. There are a number of single nucleotide polymorphisms (SNPs) that are hypothetically protective against infection by prion disease. Dogs and pigs appear resistant to prion disease, while human, cat and cow all are susceptible to prion infection.
We have shown that, although being resistant to prion disease, both dog PrP and pig PrP readily form amyloid fibrils in the test tube and the resulting fibrils are capable of enhancing amyloid fibril formation of any of the PrP sequences we tested, regardless of species origin, prion susceptible or resistant.
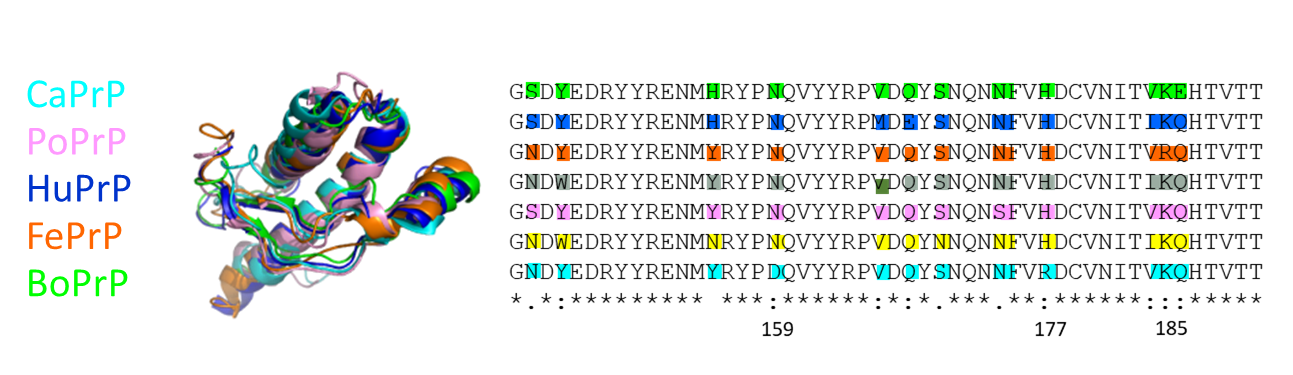 We study prion protein sequences from several different mammals and how their similarities and differences dictate their ability to form amyloid and interfere with each other. Image adapted from Nyström and Hammarström Scientific Reports 2015 https://www.nature.com/articles/srep10101
We study prion protein sequences from several different mammals and how their similarities and differences dictate their ability to form amyloid and interfere with each other. Image adapted from Nyström and Hammarström Scientific Reports 2015 https://www.nature.com/articles/srep10101
One intriguing feature about prion biology is the strain phenomenon and how it is coupled to a species barrier that moderates which species can infect and be infected by which.
Although very similar in native three-dimensional structure, PrP can misfold into numerous misfolded states and thereby cause disease with different clinical attributes as well as histologic lesion profiles. Often, the strain rather than the amino acid sequence dictates the course of disease, as well as intra- and inter species transmission. When a prion strain is transmitted from one species to another, it can reside in, and transmit between individuals of the new host species without causing clinical disease, for several generations before it eventually has adapted to its new host. The novel, adapted strain can then cause disease with different clinical symptoms than were seen in the original donor species.
 Prions strains rather than amino acid sequence dictate their infectivity. Prions are known to be able to adapt to novel species, generating new infectious strains.
Prions strains rather than amino acid sequence dictate their infectivity. Prions are known to be able to adapt to novel species, generating new infectious strains.
Since 2016 it has been known that Chronic Wasting disease is present in Europe, after the discovery of the disease in a reindeer in Norway. As of March 2019 the disease is also a fact in Sweden. Already at this early stage of investigation there are strong indications that the CWD prion strains found in Scandinavia are different from those found in North America.
We run a BSL3 lab dedicated to the studies of prions, specializing in experiments in the intersection between recombinant proteins and tissue samples.
